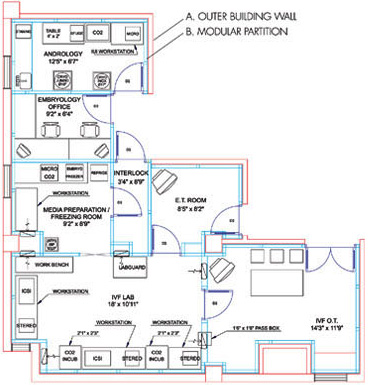
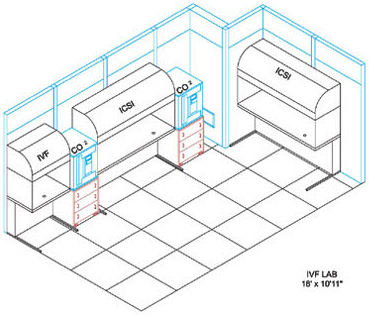
| THE IVF LAB FACILITY The IVF Lab Facility is the most critical work area in the entire ART Centre. In this area sperm embryos and Oocytes are manipulated openly The design
consideration is normally on internal air quality (IAQ) requirements. The facility
can be Grade A, Grade B, Grade C or Grade D depending on the standard followed
and implemented. SAMPLE HANDLING AND PROCESSING The IVF Lab designs
prime purpose should be to ensure the quality and safety of transplanted tissue
and cells while this means that specimen handling must be performed in a way that
they should minimize risks of infection and decease. Transmission, It must also
ensure that the processing requirements do not compromise the functional potential
of the cells. MODULAR CLEAN ROOM AND AIR QUALITY The air quality
required may vary from air grades A-D. Microbial contaminant level can be minimized
with Hepa filtration. VOC filtrations and modular clean room design. The Modular
clean room concept facilitates ease in building of partitions, ease of cleanability,
cuving of joints, provision for electrical and Gas pipelines, with proper aesthetic
looks. The IVF Lab design should address the packing of the Tissues and Cells storage, which in the case of reproductive material means cryo banking. The
IVF Lab design should also lead to the pronouncement of required standards for
traceability of specimen. This will involve labeling specification and requirement
for data retention. |
| |
|
| |||
|
| |||
|
| |||
|
| |||
|
| |||
|
| |||
|
| |||
|
| |||
|
|